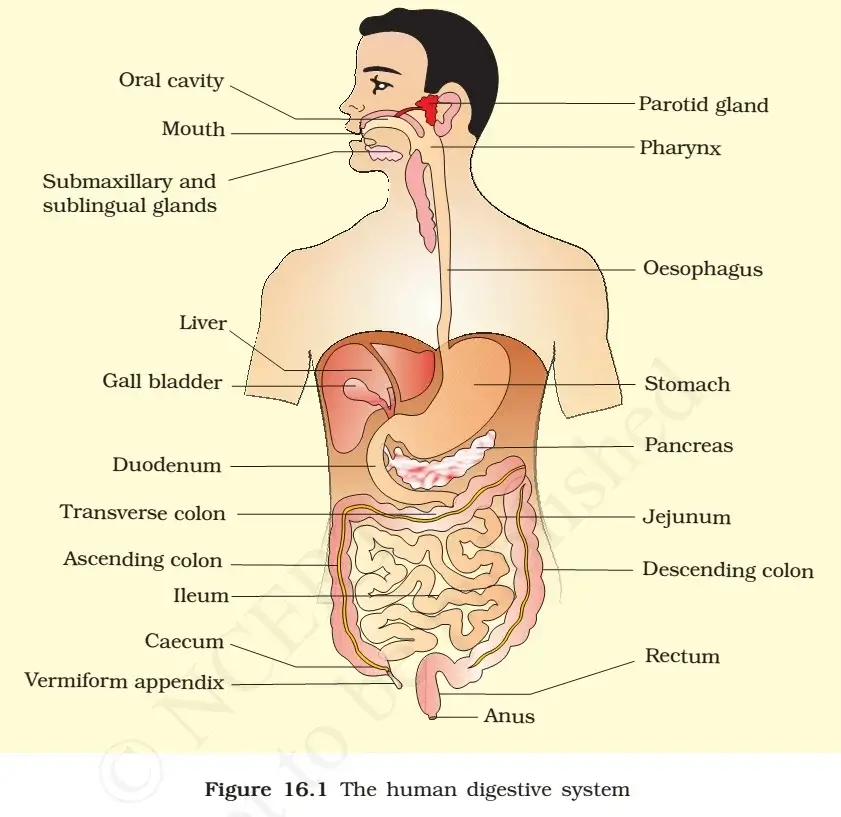
Definition of Acids and Bases
According to Arrhenius :
Acids: Substances which gives hydrogen ion in its aqueous solution. e.g. Nitric acid, HCL, Acetic acid.
Bases: Substances which gives hydroxil ion in its aqueous solution. e.g. NaOH, KOH, NH4OH.
According to Bronsted and Lowry theory:
Acids: Substances that has tendency to lose a proton.
Bases: Substances that has tendency to accept an electron.
Alkali
- An alkali is a base that dissolves in water. They are soapy to touch, bitter and corrosive. The process of dissolving an acid or a base in water is a highly exothermic one. e.g. NaOH.
- Acids and Alkalis are both electrolytes and will conduct electricity.
Properties of Acids & Bases
Acids can be defined in many ways but generally their aqueous solutions have the following properties.
- They are sour in taste.
- They turn blue litmus red.
- They react with certain metals and liberate hydrogen gas.
- They react with oxides and hydroxides of metals forming salt and water.
- Their aqueous solutions conduct electricity
Bases are defined in various ways but generally substances having the following characteristics are called bases.
- They have a bitter taste.
- Their aqueous solutions have a soapy touch.
- They turn red litmus blue.
- They react with acids to form salt and water.
- Their aqueous solutions conduct electricity.
| Acids(pH<7) | Bases(pH>7) |
| Sour in taste | Bitter in taste |
| Turns blue litmus paper red | Turns red litmus paper blue |
| React with oxides and hydroxide of metals to form salt and water | React with acid to form salt and water |
| Give off hydrogen ion when reacts with water | Give off hydroxide ion when reacts with water |
| Their aqueous solution conduct electricity | Their aqueous solution also conduct electricity |
Chemical Properties of Acid & Base
- Acid+Metal -> Salt + Hydrogen gas
- Metal carbonate/Metal hydrogen carbonate + Acid -> Salt + Carbon dioxide + Water
- Base + Acid -> Salt + Water
- Metal Oxide + Acid -> Salt + Water
Acid Base indicator
- Litmus is a natural indicator.
- Litmus solution is a purple dye, which is extracted from lichen, a plant belonging to the division Thallophyta, and is commonly used as an indicator.
- When the litmus solution is neither acidic nor basic, its colour is purple.
- Other natural indicators are:- red cabbage leaves, turmeric, coloured petals of some flowers such as Hydrangea, Petunia and Geanium, which indicates the presence of acid or base in a solution.
Red Cabbage Juice as Acid Base Indicator
- Red cabbage juice is known as an acid/base indicator because it has pigments in it that react differently to acids and bases.
- These pigment change color when exposed to an acid or a base.
- Cabbage juice is naturally neutral and it has a purplish color.
- When acid is added to it, it turns pink. If a base is added, it turns green.
- For example, the juice will turn pink when lemon juice is added to it. It changes to green when toothpaste is mixed with it, because toothpaste is basic in nature. The tooth paste should be basic due to the reason, that some acids are formed by the bacteria in our mouth and these are neutralized by the bases present in the tooth paste.
Weak and Strong acids and bases
- Acids such as Hydrochloric Acid (HCl), Sulphuric Acid (H2SO4) and Nitric Acid (HNO3) which are almost completely ionised in aqueous solution are termed as strong acids. Acids such as acetic acid (CH3COOH) is partially ionised and is called a weak acid.
- Similarly, bases like NaOH and KOH are almost completely ionised in aqueous solution and are therefore called strong bases. Ammonium Hydroxide is partially ionised and is called a weak base.
Important Notes on Acids and Bases
- Acid-base indicators are dyes or mixtures of dyes which are used to indicate the presence of acids and bases.
- Acidic nature of a substance is due to the formation of H+(aq) ions in solution. Formation of OH–(aq) ions in solution is responsible for the basic nature of a substance.
- When an acid reacts with a metal, hydrogen gas is evolved and a corresponding salt is formed. When a base reacts with a metal, along with the evolution of hydrogen gas a salt is formed which has a negative ion composed of the metal and oxygen.
- When an acid reacts with a metal carbonate or metal hydrogen carbonate, it gives the corresponding salt, carbon dioxide gas and water.
- Both acidic and basic solutions in water conduct electricity because they produce hydrogen and hydroxide ions respectively.
pH Value
How strong a given acid or base is?
We know that all the acids and bases do not react with the same chemical compound at the same rate. Some react very vigorously, some moderately while others show no reaction. To determine the strength of acids and bases quantitatively, we use a universal indicator which shows different colours at different concentration of hydrogen ion in solution. Generally, the value of pH of acids and bases are used to quantitatively determine their strength.
- It can be known by making use of a universal indicator, which is a mixture of several indicators.
- The universal indicator shows different colours at different concentrations of hydrogen ions in a solution.
- A scale for measuring hydrogen ion concentration in a solution, called pH scale.
- P in pH stands for potenz in German, meaning power.
pH should be thought of simply as a number which indicates the acidic or basic nature of a solution. Higher the hydronium ion concentration, lower is the pH value.
pH is defined as the negative logarithm of H+ ion concentration. Hence the meaning of the name pH is justified as the power of hydrogen.
pH = – log10 [H+]
For pure water and neutral solutions, at 298 K, concentration of hydrogen ions is 1 x 10-7 mol L-1. These hydrogen ions are formed by ionisation of some of the water molecules.
H2O (l) ↔ H(aq) + + OH(aq) –
Thus, for pure water at 298 K,[H+] = [OH-] = 1 x10-7 mol L-1
So, pH of distilled water is :
-log (1 x 10-7) = 7
pH scale
- A pH scale is a tool for measuring acids and bases. The scale ranges from 0-14: Litmus paper is an indicator used to tell if a substance is an acid or a base. The colour of the paper matches up with the numbers on the pH scale to indicate what kind of substance is being tested. For example, Vinegar is an acid and measures 2.4 on the pH scale.
- A healthy pH balance plays a significant role in your overall well being, and doctors and scientists usually agree on this. The pH level, or possible level of hydrogen, in your body is determined by the food and type of drink you consume. The pH is the concentration of the hydrogen ions. This calculation is based on a 0 to 14 scale.
- The pH scale is logarithmic and shows the solution’s concentration of hydrogen ions inversely. This is because the formula used to measure pH approximates the molar concentration of hydrogen ions in the solution to the negative of the base 10 logarithms. More specifically, pH is the negative of the activity of the H+ ion from the base 10 logarithms.
- The pH scale can be traced to a series of standard solutions whose pH is defined by international agreement. By calculating the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode, primary pH standard values are calculated using a concentration cell with transference.
- A glass electrode and pH metre, or colour-changing indicator, may be used to measure the pH of aqueous solutions.
pH Value and Nature of a Solution
- If [H+] > 10-7, pH is less than 7 and the solution is acidic.
- If [H+] = 10-7, pH is 7 and the solution is neutral.
- If [H+] < 10-7, pH is more than 7 and the solution is basic.
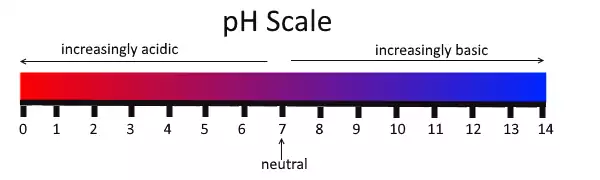
On the pH scale:
0 -> very acidic
7 -> neutral
14 -> very basic
pH Value for some familiar solutions :
| pH Value (Approximate) | Example |
| 0 | battery acid, hydrochloric acid |
| 1 | gastric acid |
| 2 | lemon juice, vinegar |
| 2.5 – 3.5 | grapefruit juice, soda, tomato juice |
| 3 | Soft Drinks |
| 4 | acid rain |
| 5 | black coffee, bananas |
| 6.3-6.6 | urine, milk, saliva |
| 7 | pure water |
| 7.4 | blood |
| 8 | sea water, eggs |
| 9.5 | baking soda |
| 10 | Great Salt Lake, milk of magnesia |
| 11 | ammonia solution |
| 12 | soapy water |
| 13.5 | bleach, oven cleaner |
| 14 | liquid drain cleaner |
Importance of pH
- Only a narrow range of pH change can be sustained by a living organism, any further change in pH can make the living difficult. For example: in the case of acid rain, the pH of water is less than 7. As it flows into a river, it lowers the pH of river water which makes the survival of aquatic life difficult.
- We know that our stomach contains hydrochloric acid which helps in the digestion of food. When the stomach produces too much of hydrochloric acid during indigestion, we feel a lot of pain and irritation. Hence, we generally use antacids or a mild base which increases the pH of the acidic stomach and thus decreases the pain.
- Bacteria present in our mouth sometimes lower the pH of our mouth by producing acids through degradation of the food particle. Hence, we are instructed to clean our mouths with toothpaste (which are generally basic) to prevent their decay by maintaining the pH.
- We experience a lot of pain in case of bee-sting as the bee injects the methanoic acid through its sting. Hence, we are generally advised to apply baking soda or other mild bases on the surface as it helps in maintaining the pH of the surface.
Points to Remember on pH Scale
- pH of strong acid or base does not depend upon temperature.
- pH of weak acid decreases with increase in temperature due to increase in ionization.
- pH of weak base increases with increase in temperature due to increase in ionization or [OH-] ion concentration.
Why does a Water Source Change pH?
- Surface water usually has a pH value of 6.5 to 8.5 and groundwater appears to have a pH of 6.0 to 8.5. The pH of a source of water can naturally vary. Some types of rock and soil, such as limestone, can more effectively neutralize acid than other rock and soil types, such as granite.
- Or, when large numbers of plants grow in a lake or river, when they die and decompose, they release carbon dioxide. A weak carbonic acid is produced when the carbon dioxide interacts with the water; this can then cause the water body to decrease its pH.
With an Increase in Temperature pH of Pure Water
- As temperature increases the degree of dissociation of water increases, thus water dissociates to give more [H+], and hence its pH decreases.
- Temperature plays a significant role on pH measurements. As the temperature rises, molecular vibrations increase which results in the ability of water to ionize and form more hydrogen ions. As a result, the pH will drop.
- pH decreases with increase in temperature. In the case of pure water, there are always the same concentration of hydrogen ions and hydroxide ions and hence, the water is still neutral (even if its pH changes). At 100°C, a pH value of 6.14 is the new neutral point on the pH scale at this higher temperature.
Also refer: